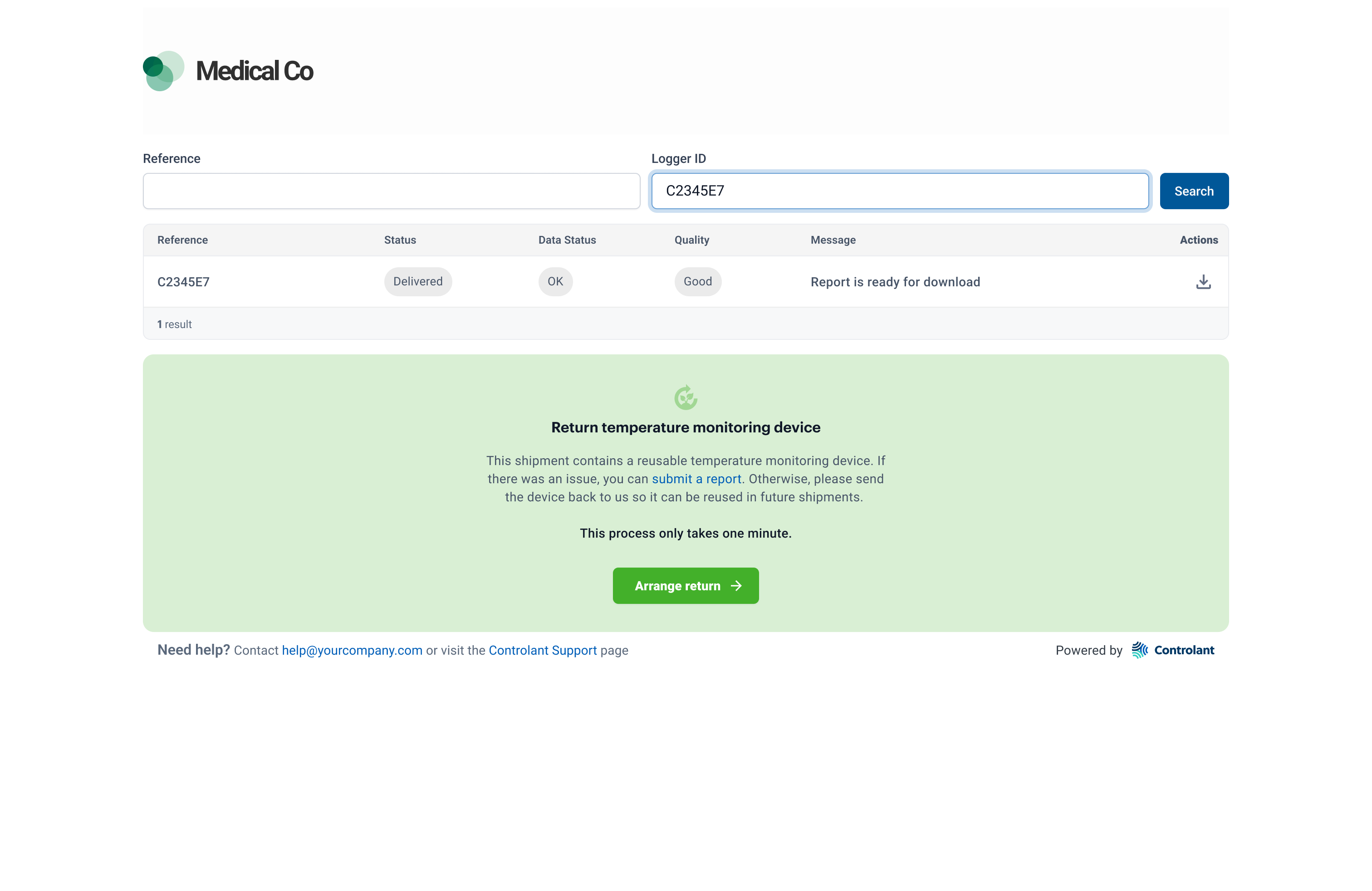
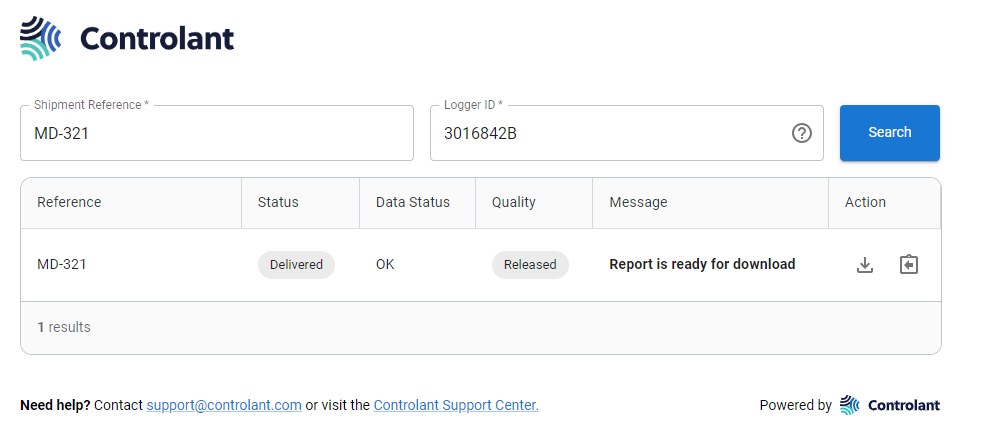
Searching for a shipment
When a delivery site receives a a shipment from a pharma company, delivery site representatives can instantly check to see whether the pharmaceuticals are safe to use, via a web site provided by the pharma company.
If the Quality Report is available, you can download it.
Click
 .
.A pdf file of the Quality Report is downloaded. You may be asked to select the folder to save the file in.
Navigate to the folder you saved the report.
Open the report.
For more information about the report, see Quality Report.
If the Quality Report quality report is not available, and you get the message Report not yet available, you can sign up to receive an email notification when the Quality Report becomes available.
Requesting notification email when Quality Report is available:
To sign up to receive an email notification when the Quality Report is available, click
 .
.The Subscribe dialog appears:
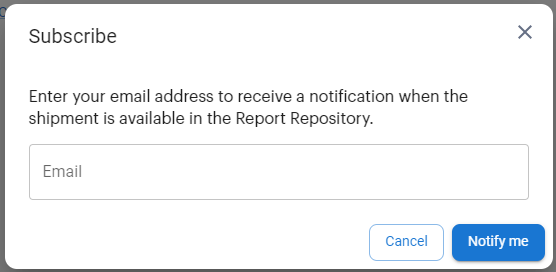
In the Email box, type your email address.
Click Notify me.
When the report is available, you will receive an email notification containing a link to a Share Portal page where you can download the report.
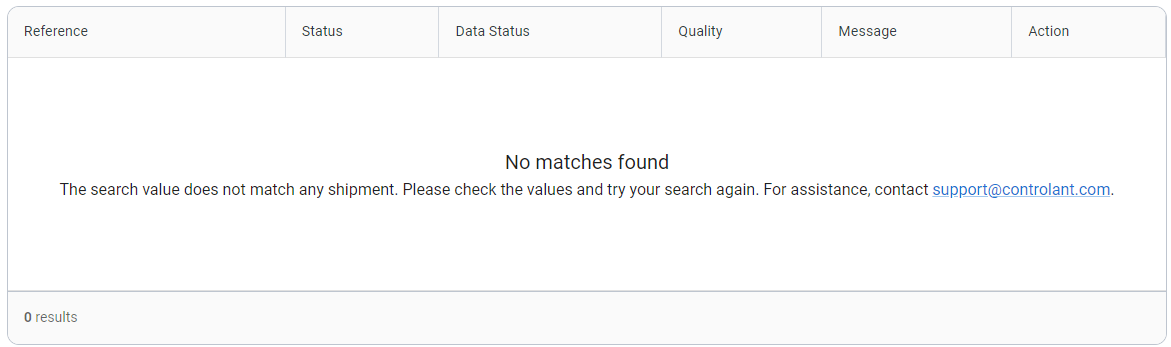
If no match is found for your search parameters, check whether there is a typo in the parameters you supplied and try again.
Contact support@controlant.com.